Emily Willingham
Much of what I saw at IMFAR (self-selected, obviously) focused on assessing sex hormone differences or the presumed outcomes of such differences in autistic vs non-autistic populations. As the Father of the Extreme Male Brain Hypothesis that androgen levels relate to autism, Simon Baron-Cohen appeared as senior author on several posters in this subject area and also gave a talk on the same topic. While he is possibly best known in a negative light in autism circles for his tautological “autistic people do poorly on my empathy test ergo autistic people lack empathy” ideas, what I discuss below is not related to that, at all. It’s all about the steroid hormones during development in the womb, and I found it fascinating — again, self selection as someone whose research focus was hormones during development. I’m not the only TGPA editor to have an interest in the link between steroid hormones and autism; editor Carol Greenburg has described her personal connection to it, as well.
If you’re not familiar with the androgen hypothesis of autism, a brief explainer is in order. Some endpoints of exposure to androgen in the womb appear to be more frequent among autistic people. In addition, there is a presumed bias to males in the autistic population (although I’ll continue to argue that autistic girls and women are likely to be overlooked far more often than autistic boys and men). Among these endpoints are traits like a “masculinized” digit ratio, in which the ring or fourth finger is longer than the index or second finger. The lower this ratio (longer ring finger), the more masculinized the hand in question is. The average of this ratio among women is near 0.97, and for men, it is 0.947. My own digit ratio is a whoppingly low 0.93 (yes, I have masculinized hands). Some researchers have found that digit ratios in autistic people are lower — more masculinized — than they are in non-autistic people, although results on that are mixed.
 |
| Source: Government Image |
Given this and some other features related to androgen exposure in the womb, Baron-Cohen’s research group has focused on assessing exactly what that exposure might have been for people who are autistic compared to people who are not. It’s not easy to gain access, especially in retrospect, to conditions in the womb, but thanks to the Danes and their obsession with tracking their population’s health parameters, just such access is possible. They happen to maintain a biobank of amniotic fluid samples acquired from amniocentesis. Thanks to their tracking, they also know whether the children resulting from those pregnancies later were diagnosed with autism.
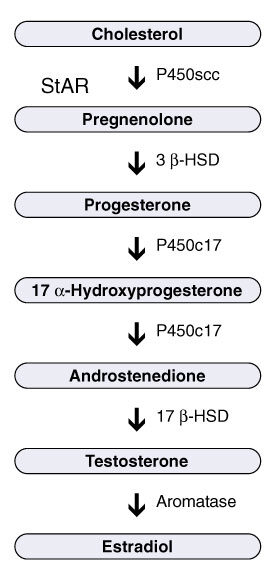
For this study, the researchers had 62 samples pregnancies that resulted in children later diagnosed with “classic” autism or Asperger syndrome and 231 samples from children who were later not diagnosed with anything, so who were presumably neurotypical. Using sensitive techniques, the investigators analyzed the samples for a series of hormones, several from the pathway that ultimately leads to production of testosterone. The androgen pathway starts with a hormone called progesterone. Thanks to the activity of enzymes, this pathway then steps through two other hormones before finally ending with production of testosterone. They used cortisol, the “stress hormone,” as a control hormone that’s made from progesterone via a different pathway.
Baron-Cohen’s team found higher concentrations of all four hormones in the androgen pathway among samples associated with autistic people compared to those associated with non-autistic people. They did not find differences in cortisol levels between the two groups — leading me to wonder, does this point away from the “stress” hypothesis of autism? So many hypotheses, so little time.
In presenting these data, Baron-Cohen was careful to stress that replication would be needed to confirm the findings. Indeed, the design does have issues, likely constrained by how difficult it is to find amniotic fluid samples that are well stored and that can be linked to post-natal outcomes. One of the most glaring problems is that the samples came from pregnancies between 10 and 20 weeks of gestation. That’s a huge range that entails changes in hormone levels throughout that time period. That said, the lack of difference in cortisol levels between the groups does help to mitigate that limitation somewhat. The cortisol results lead, however, to my other concern, which is that cortisol can interact with the hormones in the androgen pathway and vice versa, so why wouldn’t cortisol differ between the groups?
The hormone party didn’t end with Baron-Cohen, however. A Swedish group led by S. Bejerot presented a poster asserting that “many adults with ASD appear androgynous, youngish for their age, and ambiguous in sexual preferences.” I didn’t get the references from this poster and cannot say what they’ve used to confirm this information. The paper appears to be published now in the British Journal of Psychiatry but lies behind a paywall.
At any rate, using this presumed androgyny as a springboard, they evaluated testosterone levels in autistic and non-autistic adults, men and women. For endpoints of masculinization, the authors measured digit ratios and head/wrist/chest/waist/hip circumferences, and recorded each participant reading a short story. Then they tested for testosterone levels and used independent observers to rate participant images and voices in terms of stereotypical sex-hormone-based characteristics, such as a low voice (androgenized) or eyes far apart (estrogenized).
They found that autistic women had bigger heads, less feminine facial features, and higher levels of testosterone compared to non-autistic women. Autistic men, on the other hand, were assessed as having less masculine body characteristics and voices, and less masculine digit ratios, even though their testosterone levels didn’t differ from levels in the non-autistic men. Finally, the subjective measure of “androgynous facial features” correlated “strongly and positively” with autistic traits in both sexes. The authors conclude that autism may be a “gender defiant disorder,” which makes it sounds strangely rebellious and slightly dangerous, like one of the Wild Bunch or James Dean.
These findings might seem like a disconnect from what Baron-Cohen’s group reported, but what happens in the womb doesn’t necessarily parallel what happens in the child or adult after birth. All of this is hazy and interesting and hormonal, but it doesn’t nail down anything unequivocally.
Speaking of the womb, I come around now to tying this all up, not with a neat little bow but into a shabby, incoherent package of little hints for where to look next. You may recall the recent, famous twin study that found a good-sized contribution of the womb environment to autism. A lot of people who think “environment” means “toxins” took that as evidence that toxins cause autism. But the “environment” in genetics means a lot of other things, including the natural “hormonal milieu” that results from maternal and fetal hormone production. And that takes me to my last presentation from IMFAR that I’d like to discuss as part of this “parade of hormones” entry about the conference.
It’s a small study, one that looked at identical twins who either shared a chorionic sac during development or who did not. The implication is that a shared sac meant a highly shared environment, while two separate sacs suggest less sharing of environmental inputs. This study, by L. Meyer and H.H. Goldsmith from the University of Wisconsin, Madison, evaluated 54 pairs of identical twins. At least one member of each pair was autistic. They found that the identical twins were 69% concordant (compared to 23% for fraternal twins), and that sharing a chorionic sac didn’t make it more or less likely that both twins would be autistic. They concluded that non-shared environmental factors appeared to have no influence on autism. In other words, whatever the twins experienced in separate sacs — presumably including hormonal interaction between mother and child — didn’t differ from what they experienced when they shared a sac.
Altogether, these findings make hormones and the womb environment an interesting place to look if your thing is genetics, causes of autism, and “where did I come from, why am I like this?” Nothing here is definitive. It’s just suggestive. But as I consider my own low digit ratio, androgynous face, low voice, and large head, I can’t help but relate and feel that information like this can increase at least a little what I know about myself.